Back to Studies
IMPAACT 2010 / VESTED
Phase III Study of the Virologic Efficacy and Safety of Dolutegravir-Containing versus Efavirenz-Containing Antiretroviral Therapy Regimens in HIV-1-Infected Pregnant Women and their Infants
Study Status
Participants Off Study and Primary Analysis Completed
DAIDS Number
30129
IND Number
133,438
Clinical Trials Link
Primary Protocol Team Members
Summary
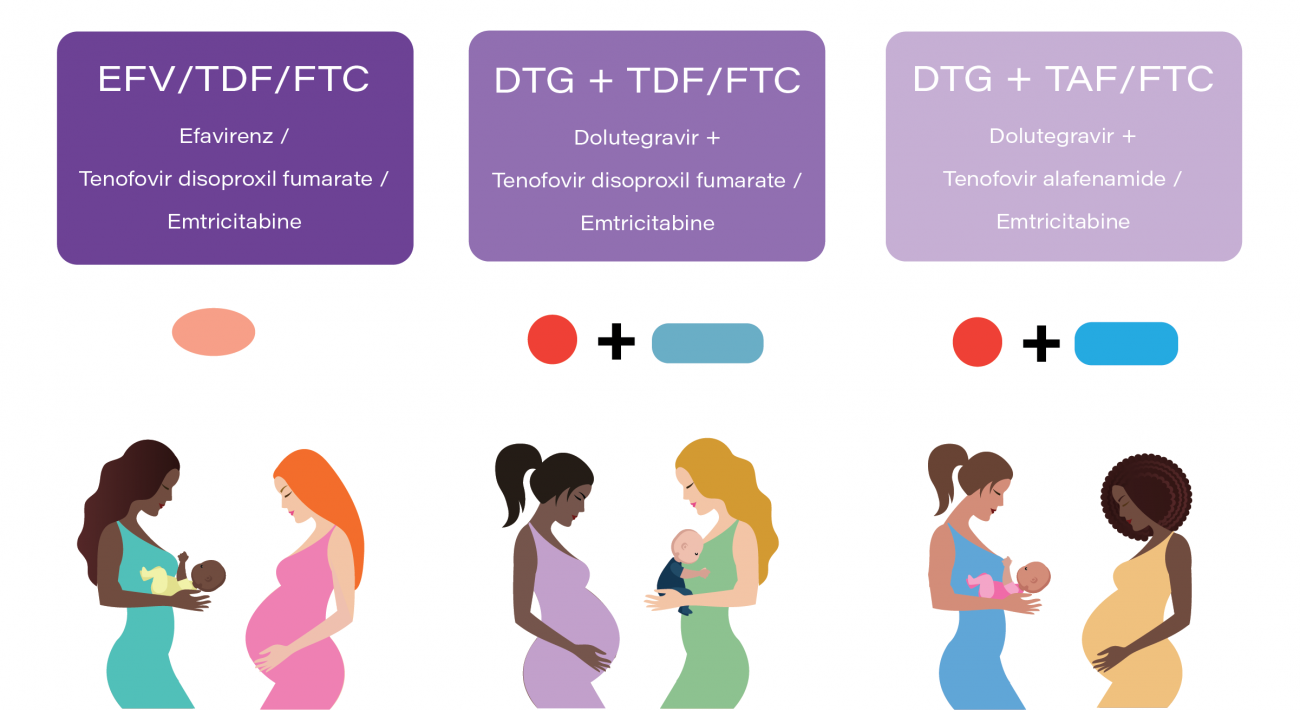
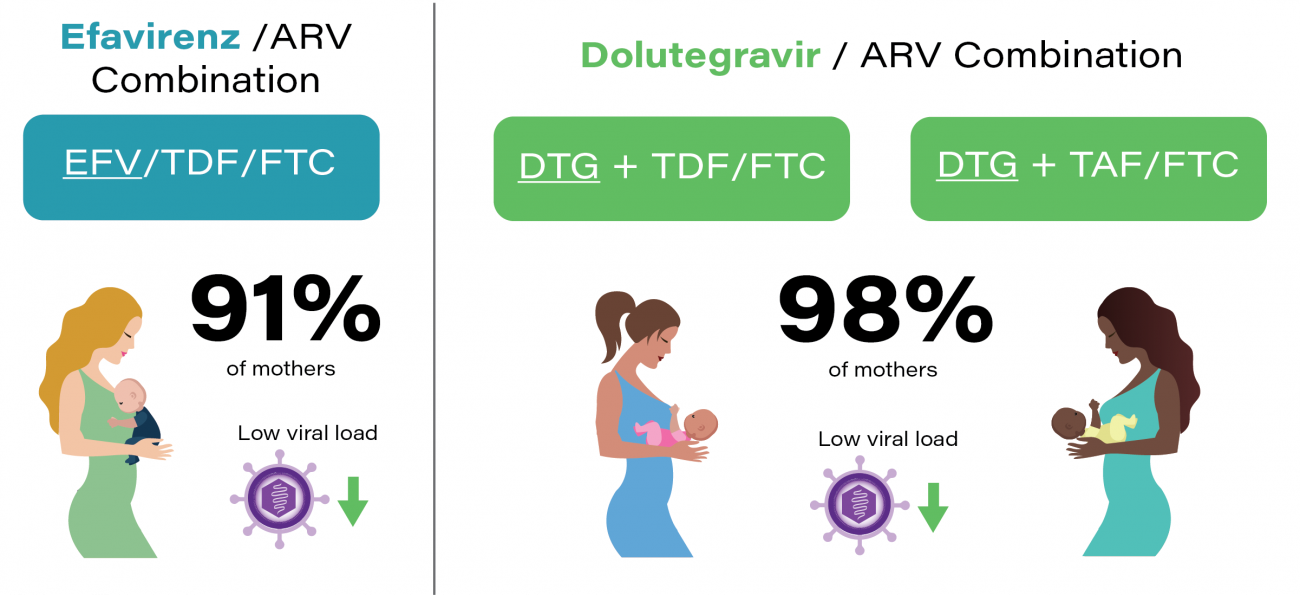
IMPAACT 2010 is a Phase III, three-arm, randomized, open-label study of HIV-1-infected pregnant women initiating either a dolutegravir-containing antiretroviral regimen or an efavirenz-containing antiretroviral regimen at 14-28 weeks gestation. The VESTED study (Virologic Efficacy and Safety of ART Combinations with TAF/TDF, EFV, and DTG) will compare the regimens with regard to safety and virologic efficacy during pregnancy and through 50 weeks of maternal and infant follow-up postpartum.
Loading study documents...
Loading study sites...
Loading study protocol team...
Loading study press...
Loading study publications/presentations...